Question 1:
Diagram 1 shows the setting up of apparatus in an experiment.
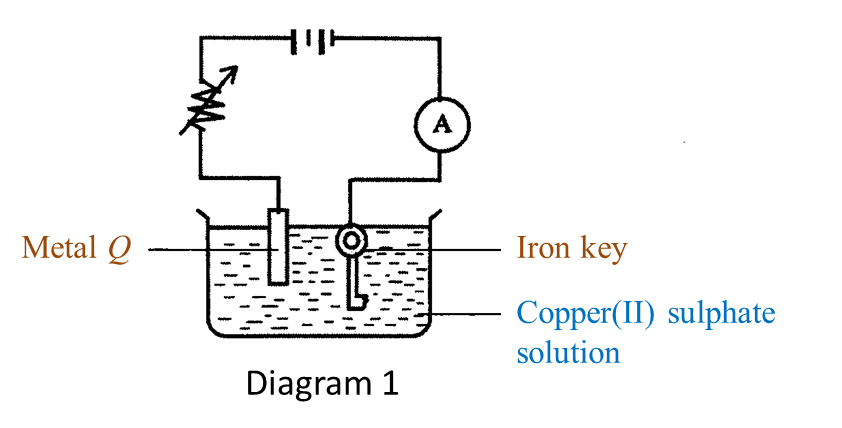 (a) Name the process in Diagram 1. [1 mark]
(a) Name the process in Diagram 1. [1 mark]
(b)(i) Name metal Q. [1 mark]
(ii) What happens to metal Q during the process in Diagram 1? [1 mark]
(c) Which metal functions as the cathode? [1 mark]
(d)(i) What will happen to the iron key at the end of the experiment? [1 mark]
(ii) State one method to get a good result in (d)(i) [1 mark]
Answer:
(a) Electroplating
(b)(i) Copper
(b)(ii) Metal Q dissolves in the copper(II) sulphate solution to form copper ions and becomes thinner.
(c) Iron key.
(d)(i) The surface of the iron key will be coated with a brown layer of copper.
(d)(ii) The surface of the metal to be plated must be cleaned with sandpaper before electrolysis begins.
Diagram 1 shows the setting up of apparatus in an experiment.
 (a) Name the process in Diagram 1. [1 mark]
(a) Name the process in Diagram 1. [1 mark](b)(i) Name metal Q. [1 mark]
(ii) What happens to metal Q during the process in Diagram 1? [1 mark]
(c) Which metal functions as the cathode? [1 mark]
(d)(i) What will happen to the iron key at the end of the experiment? [1 mark]
(ii) State one method to get a good result in (d)(i) [1 mark]
Answer:
(a) Electroplating
(b)(i) Copper
(b)(ii) Metal Q dissolves in the copper(II) sulphate solution to form copper ions and becomes thinner.
(c) Iron key.
(d)(i) The surface of the iron key will be coated with a brown layer of copper.
(d)(ii) The surface of the metal to be plated must be cleaned with sandpaper before electrolysis begins.