Half-life
- The half-life (T½ ) of a quantity subject to exponential decay is the time required for the quantity (mass, number of atom or activity) to decay to half of its initial value.
- The decay curve of a radioactive source can be found experimentally - provided the activity of the source decreases over a practicable time period (minutes, hours or days for example).
- The corrected count-rate is then plotted against time to give the decay curve. The half-life of the source can be found from the curve as indicated above.
 |
| (Decay Curve) |
Example 1
A radioisotope has half-life of 8 hours. Initially, there were 3.6 x 1018 radioisotope atoms in a sample. How much time is taken for the number of atoms of the radioisotope to fall to 4.5 x 1017?
Answer:
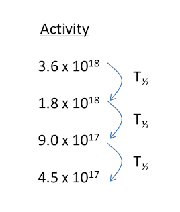 The sample take 3 half-life to decay from 3.6 x 1018 radioisotope atoms to 4.5 x 1017. Therefore, the time taken
The sample take 3 half-life to decay from 3.6 x 1018 radioisotope atoms to 4.5 x 1017. Therefore, the time taken
t = 3T½
t = 3(8) = 24h
A radioisotope has half-life of 8 hours. Initially, there were 3.6 x 1018 radioisotope atoms in a sample. How much time is taken for the number of atoms of the radioisotope to fall to 4.5 x 1017?
Answer:

t = 3T½
t = 3(8) = 24h
Example 2
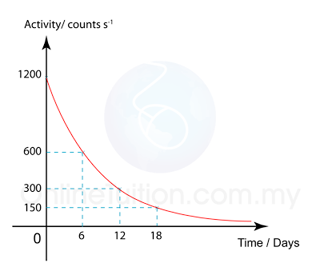
The diagram shows the graph of the activity of a radioisotope, X, against time. What is the half-life of the radioactive substance?
Answer:
The half-life is the time taken for the activity to decrease to become half of the initial value.
From the graph we can see that the radioisotope take 6 days for the activity to become half. Therefore
Half-life = 6 days

The diagram shows the graph of the activity of a radioisotope, X, against time. What is the half-life of the radioactive substance?
Answer:
The half-life is the time taken for the activity to decrease to become half of the initial value.
From the graph we can see that the radioisotope take 6 days for the activity to become half. Therefore
Half-life = 6 days