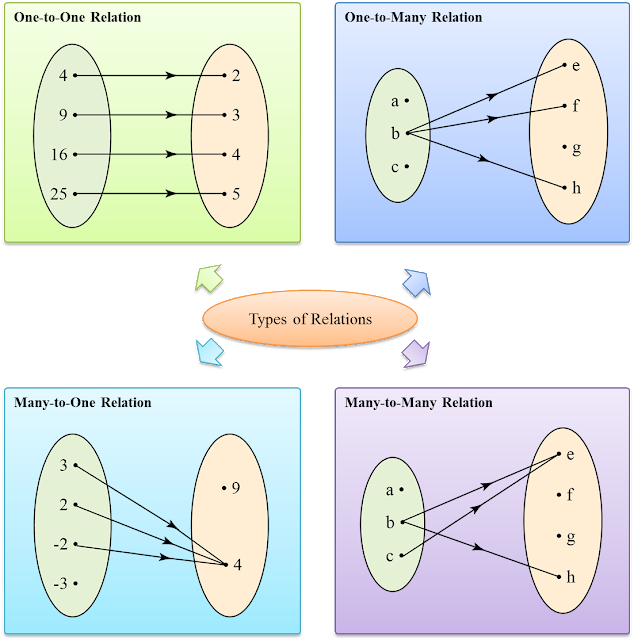
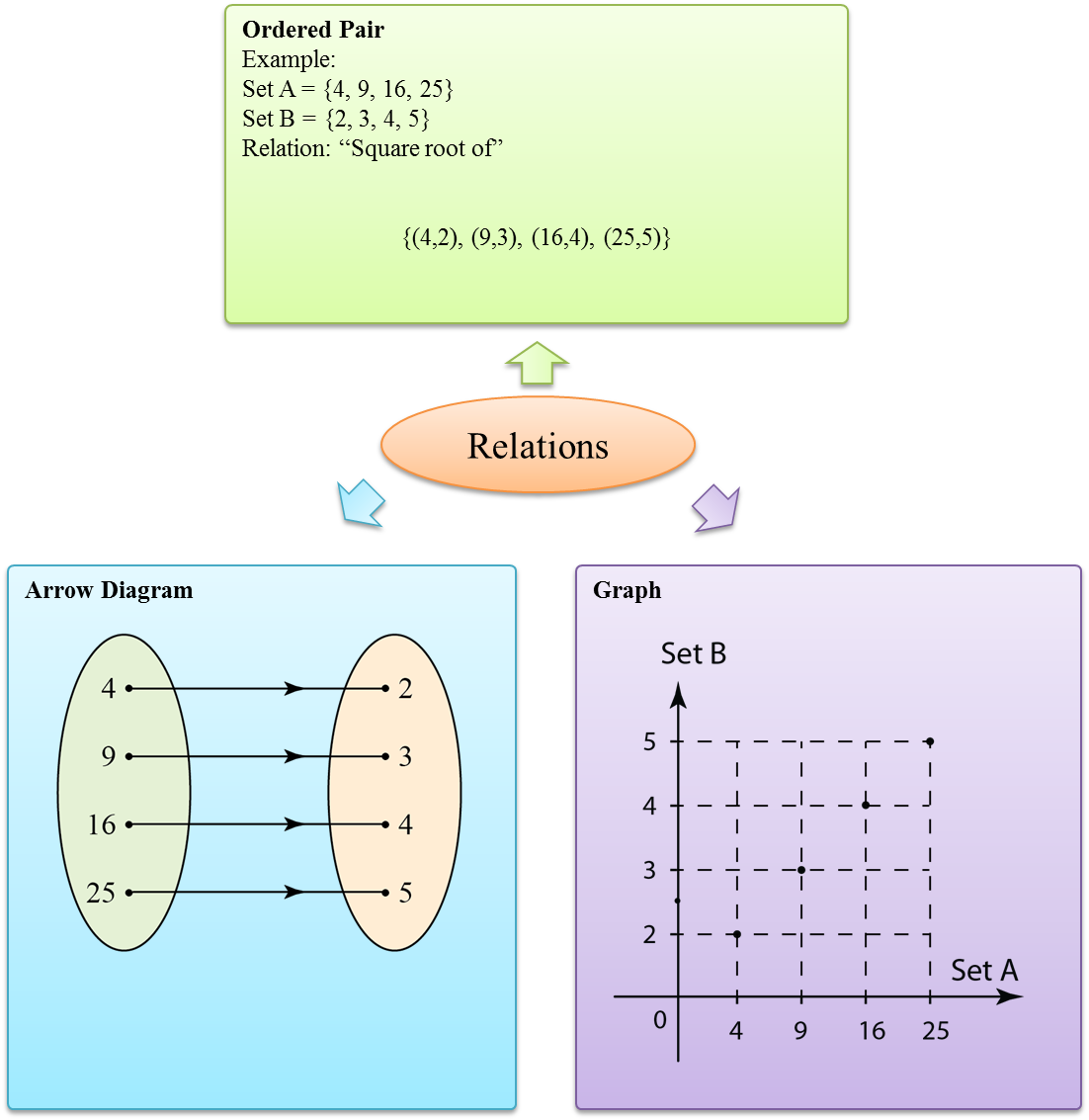
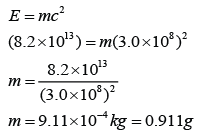- A function is a relation in which every element in the domain has a unique image (exactly one) in the codomain.
- One-to-one relation and many-to-one relation are examples of a special kind of relation which we call function.



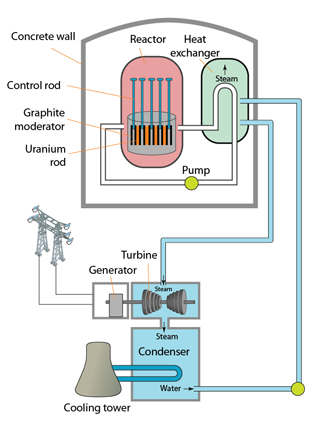
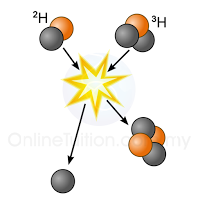
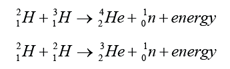
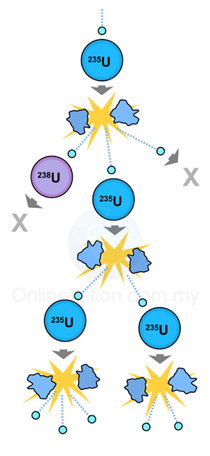 |
| (Chain Reaction) |
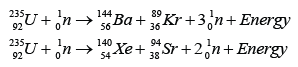
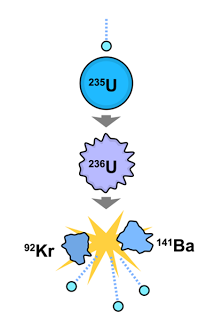
Example 1
In a nuclear reaction, the mass difference in the reaction is 1.5 x 10-8kg. Find the heat released in this reaction. [Speed of light = 3.0 x 108 ms-1]
Answer:
Mass defect, m = 1.5 x 10-8kg
Heat released,
E = mc²
E = (1.5 x 10-8)(3 x 108)
E = 1.35 x 109 J
Example 2
A nuclear explosion released 8.2 x 1013 J of energy. What is the mass defect of uranium-235 in this reaction?
[Speed of light = 3.0 x 108 ms-1]
Answer