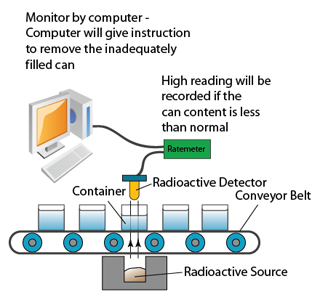
Monitoring Content of Food
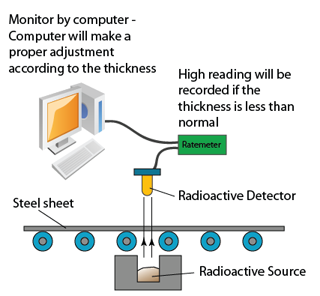
Monitoring Thickness of Steel/paper Sheet
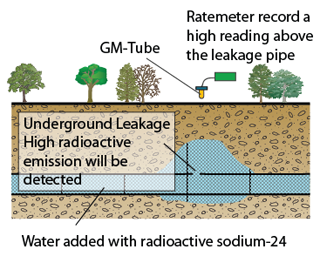
Detecting Underground Leakage
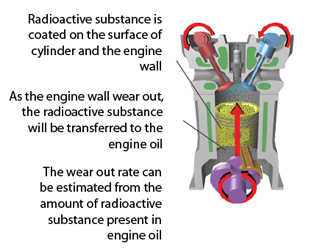
Measuring the Wearing Rate of Engine












 |
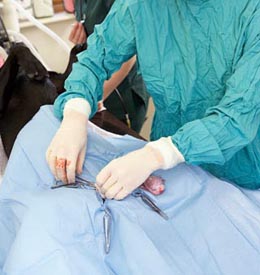
| (Heart Pacemaker) |
 |
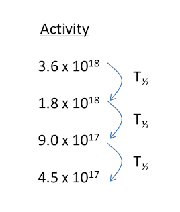
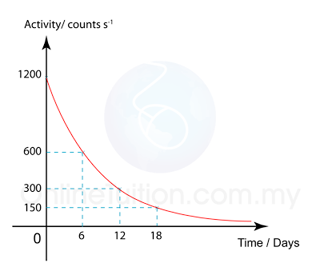
| (Decay Curve) |
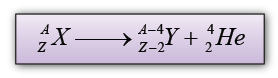
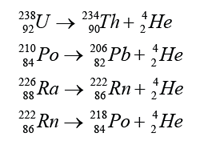
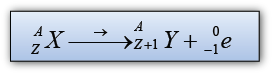
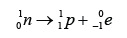
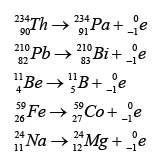
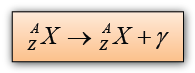



| Characteristic |
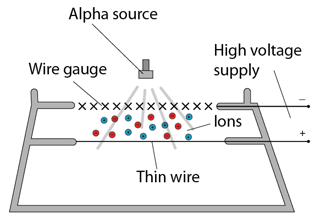
Alpha Particle
|
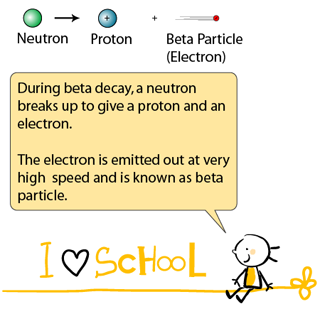
Beta Particle
|
Gamma Ray
|
| Symbol |
α
|
β
|
γ |
| Nature |
Nucleus of Helium
|
High speed electron | Electromagnetic wave |
| Charge |
+2
|
-1
|
Neutral
|
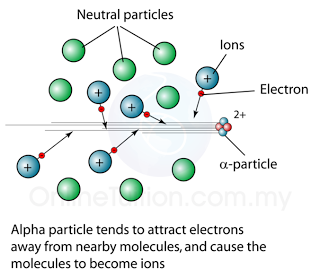
| Ionizing Effect |
strong
|
weak
|
Very weak
|
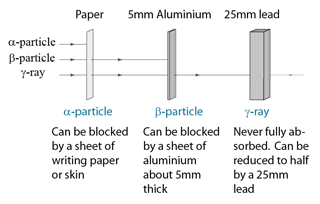
| Absorted by | Sheet of writing paper |
About 5mm of aluminium
|
Never fully absorded. 25mm of lead reduces intensity to half
|
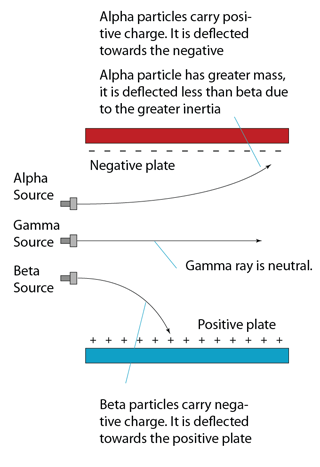
| Deflection in Electric Field |
Can be deflected
|
Can be deflected
|
Not deflected
|
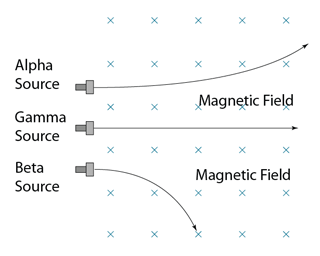
| Deflection in magnetic Field |
Can be deflected
|
Can be deflected
|
Not deflected
|
| Speed |
Up to 10% of the speed of light
|
Up to 90% of the speed of light
|
Speed of light
|
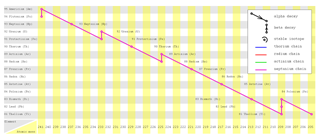 |
| (This image is created by Johantheghost under creative common licence) |
[Can detect: Alpha particles only]


[Can detect: Alpha particles only]