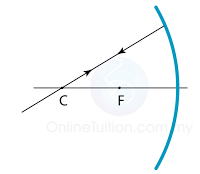
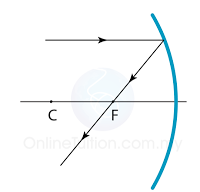
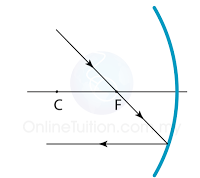
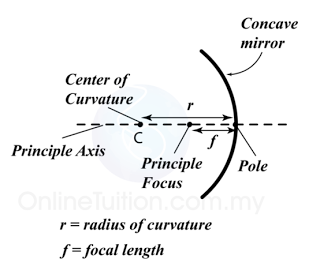
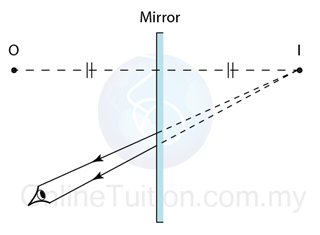
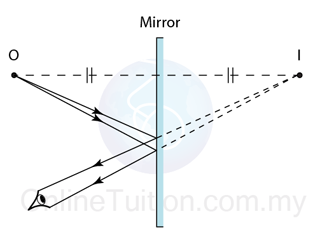
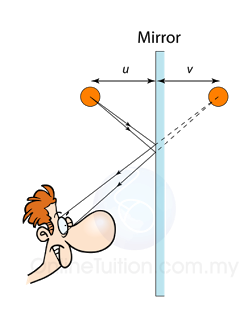
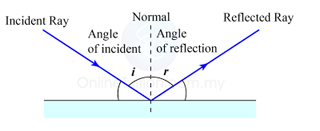
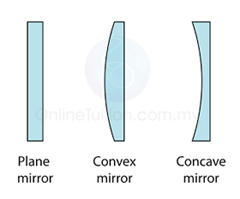
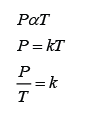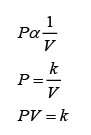Finding the Position and Size of the Image
- Any two rays are sufficient to fix the position and size of the image. Look for the point where the rays cross after reflection from the mirror.
- The interception of the two rays is the focus of the ray.
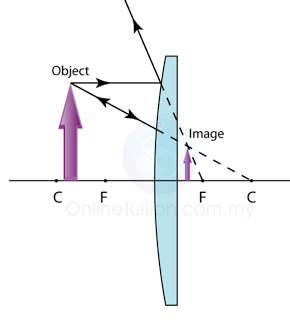
The Ray Diagram and the Types of Image
The nature and size of the image formed by concave mirrors depends on the distance of the object from the mirror. This will be explained in the following sections.