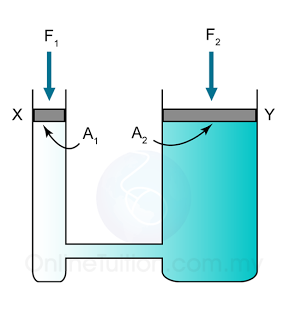
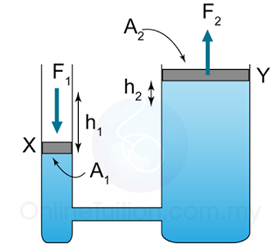
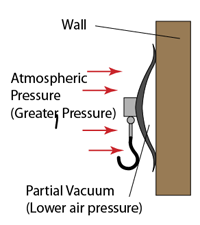
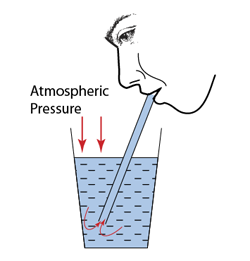
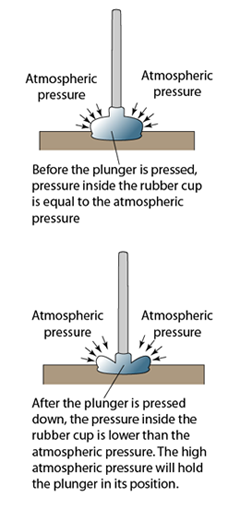
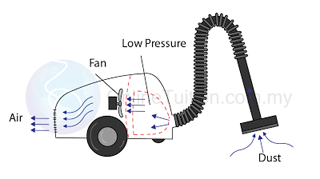
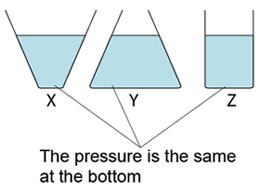
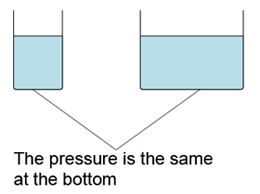
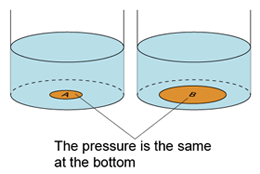
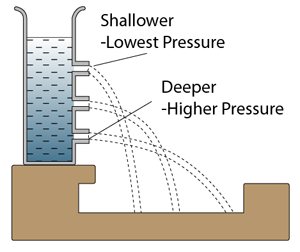
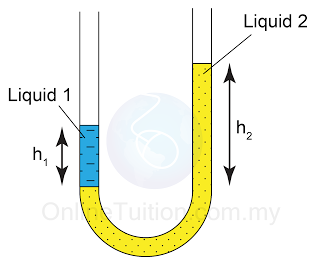
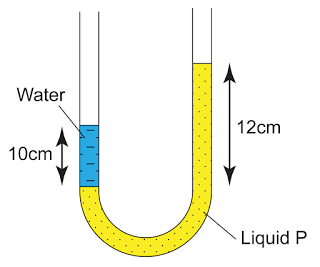
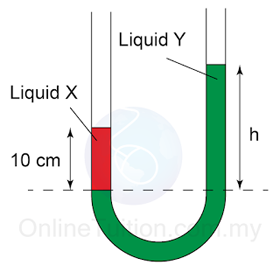
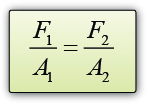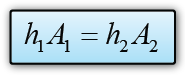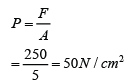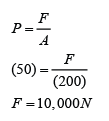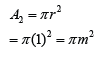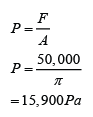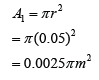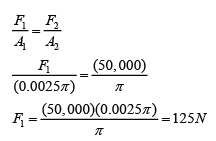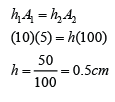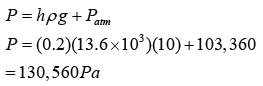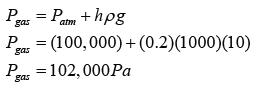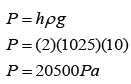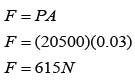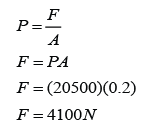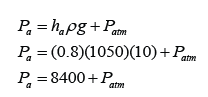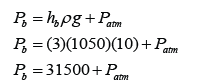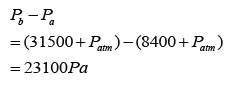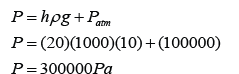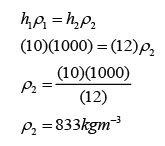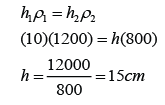- Atmospheric pressure can be measured by either
- a simple barometer,
- a Fortin barometer
- an Aneroid barometer.
- In SPM, most of the questions asked are related to the simple barometer.
- For Fortin barometer and Aneroid barometer, you only need to know their working principle.
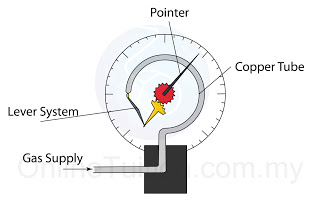
Simple Mercury Barometer
 Aneroid Barometer
Aneroid Barometer
Simple Barometer
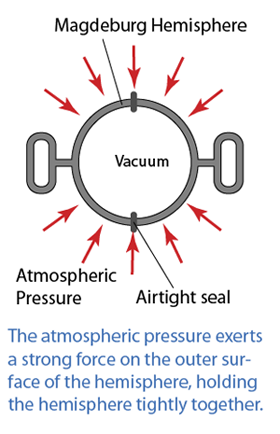
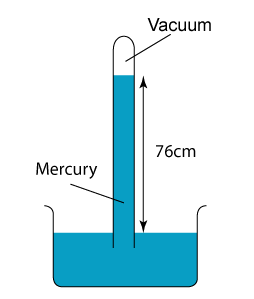
- Atmospheric pressure can be measured by a simple barometer.
- A thick glass tube (at least 1m long) is filled with mercury completely.
- The open end of the tube is covered with a finger and inverted in a trough of mercury.
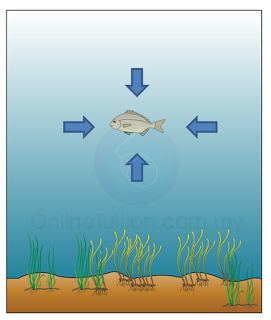
- The height of the mercury is proportional to the atmospheric pressure.
Using Simple Mercury Barometer
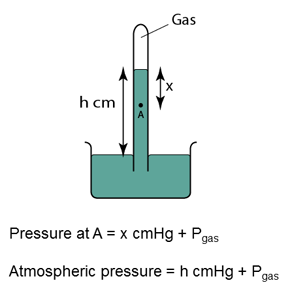
Characteristics of the Mercury Barometer
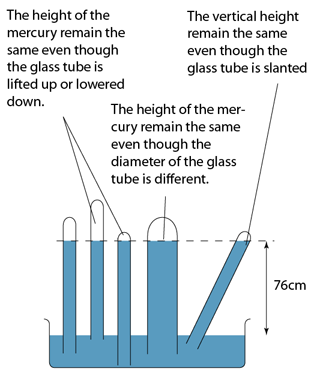
Q and A
Q: Barometer is usually made up of mercury. Explain why is it not practical to have a water barometer?
A:
- The atmospheric pressure is about 10 meter water, which means it can push the water up to 10 meter height.
- Therefore a water barometer must be at least 10 meter long.
- This is not practical because the glass tube of the barometer may be broken or topple easily. It is also difficult to keep or move such a long tube.
Fortin Barometer
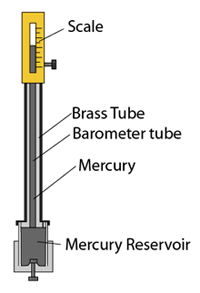
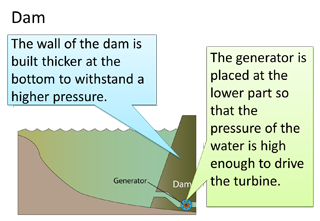
- The Fortin barometer is an improved version of the simple mercury barometer.
- The barometer tube is encased in a brass frame. This enables it to be carried around easily.
- Vernier scale is used for taking reading for extra accuracy.
Q & A
Q: What is the advantages of a Fortin Barometer over a Simple mercury barometer?
A:
- Easy to be carried around.
- More accurate.
Anaroid Barometer
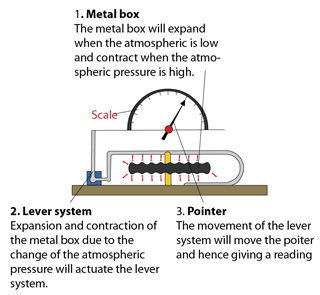
The anaroid barometer is usually used to measure altitude, which is named as altimeter.
Q & A
Q: Explain why the barometer can be used to measure altitude.
A:
The atmospheric pressure is inversely proportional to the altitude. The altitude can be determined from the atmospheric pressure.
Q & A
Q: Why a simple barometer is not suitable to be used to measure altitude?
A:
- Hard to be carried
- Mercury is volatile. It can evaporate easily
- Mercury is poisonous
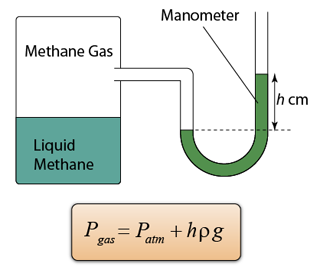
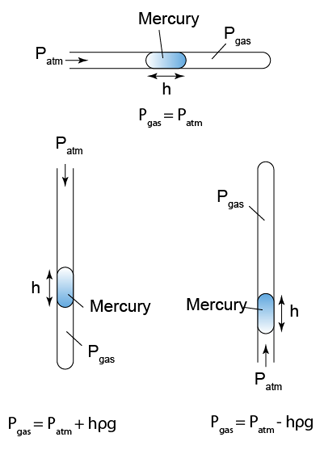
Converting the Unit from cmHg to Pa
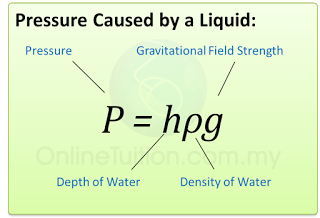
Pressure in unit cmHg can be converted to Pa by using the formula
P = hρg
Example 1:
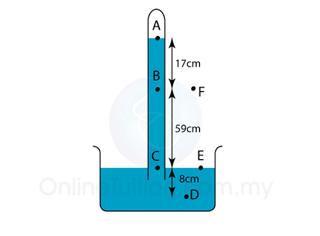
Find the pressure at point A, B, C, D, D, E and F in the unit of cmHg and Pa. [Density of mercury = 13600 kg/m³]
Answer:
| Pressure in unit cmHg |
Pressure in unit Pa |
PA = 0
PB = 17 cmHg
PC = 17 + 59 = 76 cmHg
PD = 76 + 8 = 84 cmHg
PE = 76 cmHg
PF = 76 cmHg |
PA = 0
PB = hρg = (0.17)(13600)(10) = 23,120 Pa
PC = hρg = (0.76)(13600)(10) = 103,360 Pa
PD = hρg = (0.84)(13600)(10) = 114,240 Pa
PE = hρg = (0.76)(13600)(10) = 103,360 Pa
PF = hρg = (0.76)(13600)(10) = 103,360 Pa |
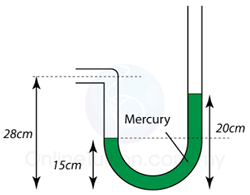
Example 2:
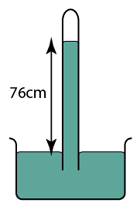
Figure above shows a simple mercury barometer. What is the value of the atmospheric pressure shown by the barometer? [Density of mercury = 13600 kg/m³]
Answer:
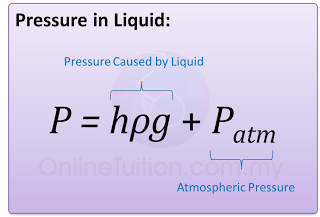
Atmospheric Pressure,
P = 76 cmHg
or
P = hρg = (0.76)(13600)(10) = 103360 Pa
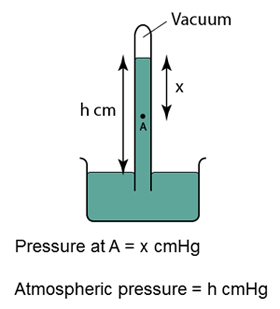
Example 3:
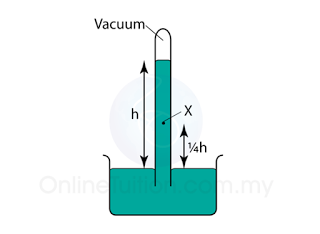
In above, the height of a mercury barometer is h when the atmospheric pressure is 101 000 Pa.
What is the pressure at X?
Answer:
Atmospheric Pressure,
P
atm = h cmHg = 101 000 Pa
Pressure at X,
P
X = (h - ¼h) = ¾h cmHg
P
X = ¾ x 101 000 = 75 750 Pa
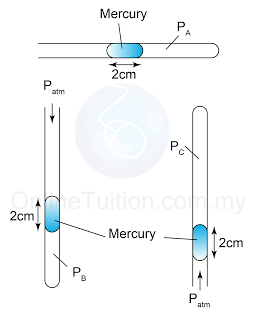
Example 4:
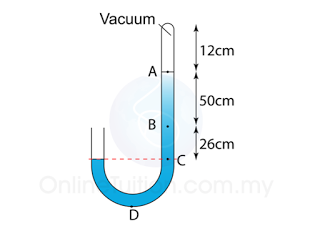
Figure above shows a mercury barometer whereby the atmospheric pressure is 760 mm Hg on a particular day. Determine the pressure at point
a. A,
b. B,
c. C.
[Density of Mercury = 13 600 kg/m³]
Answer:
a.
PA = 0
b.
PB = 50 cmHg
or
PB = hρg = (0.50)(13600)(10) = 68 000 Pa
c.
PC = 76 cmHg
or
PC = hρg = (0.76)(13600)(10) = 103 360 Pa
Example 5:
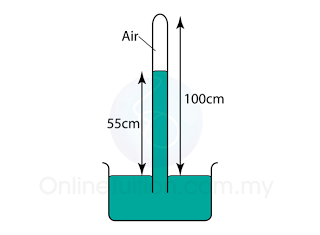
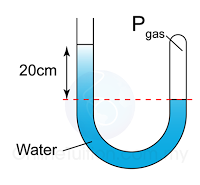
Figure above shows a simple barometer, with some air trapped in the tube. Given that the atmospheric pressure is equal to 101000 Pa, find the pressure of the trapped gas. [Density of Mercury = 13 600 kg/m³]
Answer:
Pressure of the air = Pair
Atmospheric pressure = Patm
Pair + 55 cmHg = Patm
Pair
= Patm - 55 cmHg
= 101 000 - (0.55)(13 600)(10)
= 101 000 - 74 800
= 26 200 Pa
Example 6:
If the atmospheric pressure in a housing area is 100 000 Pa, what is the magnitude of the force exerted by the atmospheric gas on a flat horizontal roof of dimensions 5m × 4m?
Answer:
Area of the roof = 5 x 4 = 20 m²
Force acted on the roof
F = PA
F = (100 000)(20)
F = 2,000,000 N
Example 7:
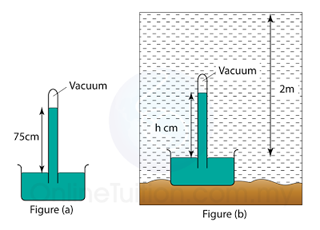
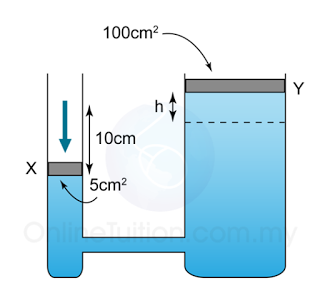
Figure(a) above shows the vertical height of mercury in a mercury barometer in a laboratory. Figure(b) shows the mercury barometer in water at a depth of 2.0 m.
Find the vertical height (h) of the mercury in the barometer in the water. Given that the pressure at a depth of 10 m from the water surface is 75 cmHg. [Density of water = 1000 kg/m³, Density of mercury = 13 600 kg/m³]
Answer:
Atmospheric pressure,
P
atm = 75 cmHg
Pressure caused by the water,
P
water = 2/10 x 75 = 15cmHg
Pressure in 2m under water
= 75 + 15 = 90 cmHg
Vertical height of the mercury = 90cm