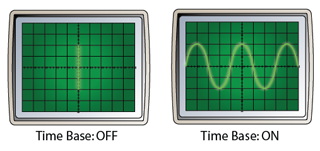
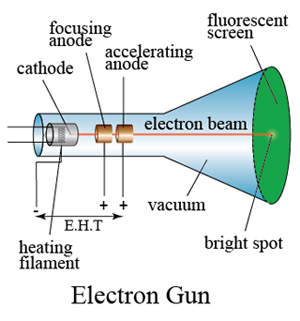
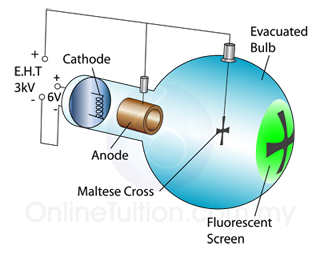
The frequently asked questions related to this topic are: what is renewable energy? the examples of Renewable and non-renewable energy and the advantages of using renewable energy.
Renewable energy sources are the energy sources which can be continuously replaced. Examples of renewable energy are:
- Hydro energy
- Solar energy
- Wind energy
- Geothermal energy
- Biomass
- Tidal energy
Advantages of Using Renewable Energy
1. Clean and do not pollute the environment.
2. Easily obtain.
3. Can be replenished once it has been used.
4. Can prevent energy crisis.
Hydroelectric

Advantages
- It is a renewable energy.
- Building a dam does not pollute the environment.
- In a lot of country, water can be easily obtained and is free.
- Building a hydroelectric plant does not involve very high technology as nuclear power plant.
Disadvantages
- Building a dam will cause a large area flooded with water, and hence seriously destroys the ecosystem nearby.
- The flooded area causes the loss of wild life habitat and agriculture land.
- Dam failure happens will cause a disaster to the lower reaches area of the river.
- The cost to build a dam is very high.
Fossil Fuel.
Advantages
- It is relatively easy to be collected as they are present in large amount in one place.
- It contains high energy capacity.
Disadvantages
- It releases a lot carbon dioxide when it is burned. Large amount of carbon dioxide presence in the atmosphere will cause the increase of the temperature of the atmosphere, known as global warming. Global warming is the most serious environmental problem that we are facing now.
- Fossil fuels are non-renewable energy.
- Pollute the air. Burning of fossil fuel will release particles and some hazardous gases such as sulphur dioxide and nitrogen dioxide which will pollute the air.
Solar Power

Advantages
- It is a renewable energy.
- Sunlight is free and can be obtained easily.
- It does not pollute the air.
Disadvantages
- A large area is needed to install the solar plate.
- The amount of sunlight at non-tropical area depends on the season.
- The energy collected need to be store in a cell so that it can be used at the time sunlight is not present.
- Its capacity is limited by the capacity of the cell. Normally a cell has relatively low capacity.
Nuclear Power
Advantages:
- The nuclear fuel such as uranium and plutonium has high energy capacity. Small amount of uranium can produces huge amount of energy.
- Nuclear energy does not pollute the air.
Disadvantages
- Hazardous radioactive waste is produced during nuclear reaction.
- The radioactive waste will cause pollution in a very large area if leakage happens in the nuclear power plan.
- The cost to maintain a nuclear plant is very high.
- Building a nuclear plant involving very high technology. Only a few countries own such technology.
Biomass
- Biomass is biological material derived from living organisms such as wood and animal waste.
- As a renewable energy source, biomass can either be used directly via combustion to produce heat, or indirectly after converting it to various forms of biofuel.
- Heat energy produced from the burning of biomass can be used to generate electricity.
Advantages
- It is renewable energy.
- Using biomass to generate electricity can reduce the disposal of organic waste.
- Liquid biofuels will not pollute the air because they are lead-free and sulphur-free.
Disadvantages
- Large storage space is required to store the biomass.
- Land utilization can be considerable. Can lead to deforestation.
- Overall process can be expensive
Wind Power

[This image is shared by Wagner Christian. This file is licensed under the Creative Commons Attribution ShareAlike 2.5 License. ]
Advantages
- It is a renewable energy.
- It does not pollute the environment.
- It is suitable for mountain area because the wind speed in mountain area is relatively high.
Disadvantages
- A large area is needed to build the windmills.
- The windmills will produce high level of noise.
- The power generated is inconsistent due to the inconsistent of the wind speed and wind direction.