Classification of Substances
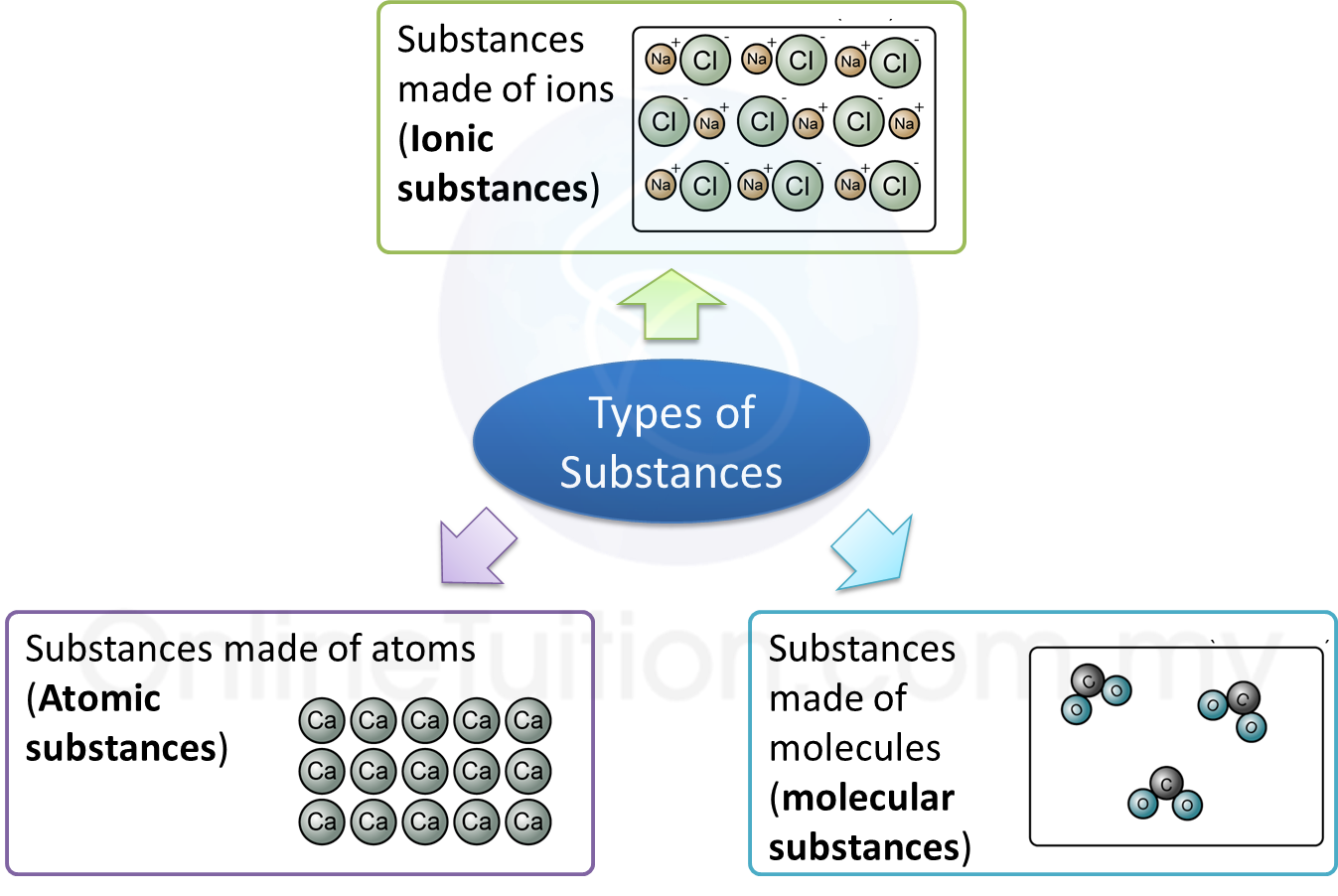
1. Types of substances:
2. The physical properties of substances depend on
(a) types of particles they contain,
(b) arrangement of the particles,
(c) forces of attraction between the particles.
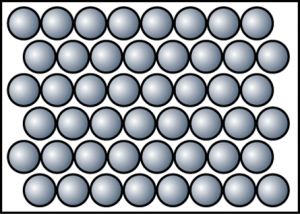
Substances made of atoms (or Atomic substances)
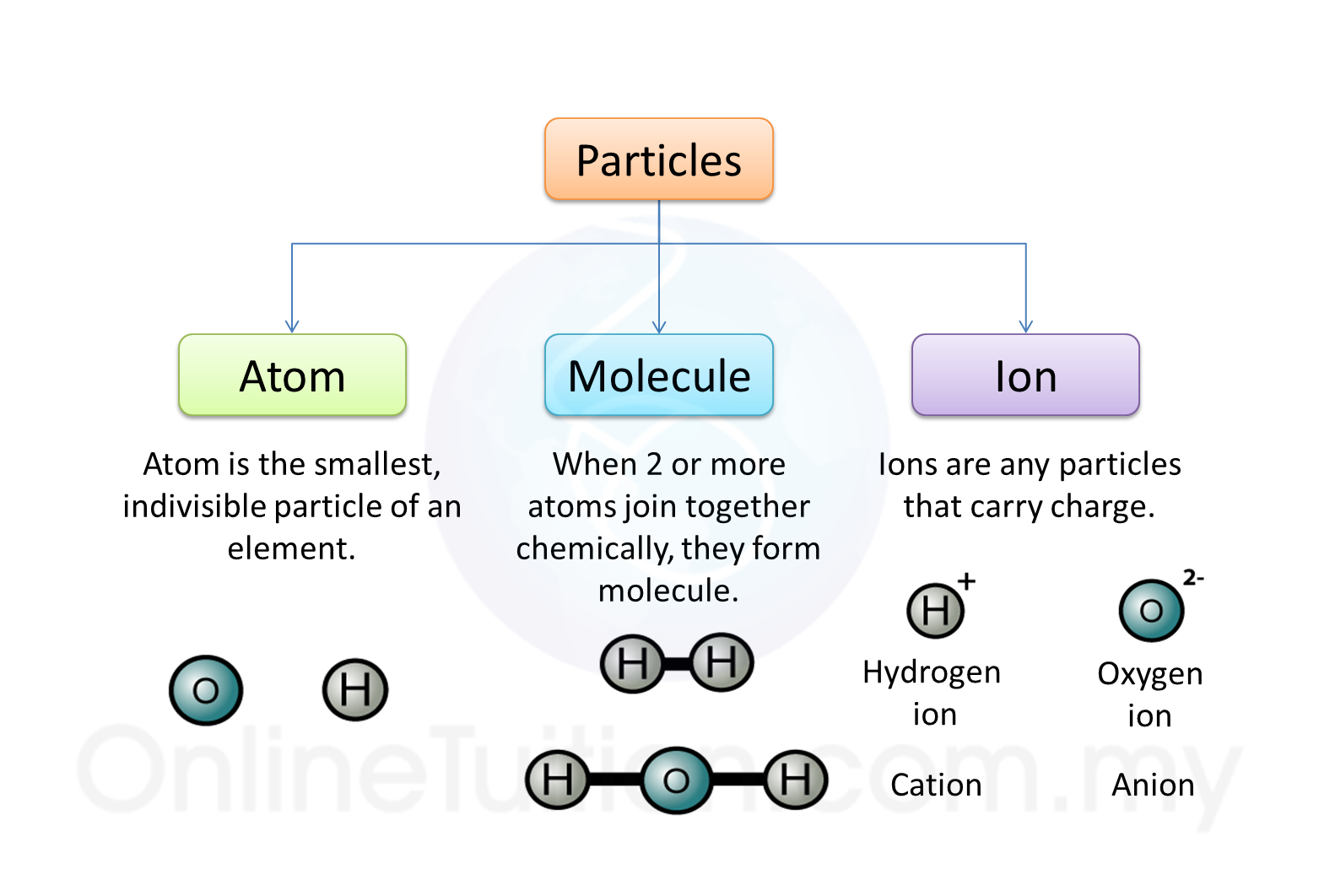
1. Atomic substances are substances that consist of only atoms.
2. The atoms in atomic substances are arranged closely together in a fixed pattern and only can vibrate in their fixed positions.
3. Atomic substances have high melting and boiling points owing to strong metallic bond.
4. They are able to conduct electricity owing to presence of free electrons.
5. All metals are atomic substances such as iron, aluminium and calcium.
Substances made of molecules (or Molecular substances)
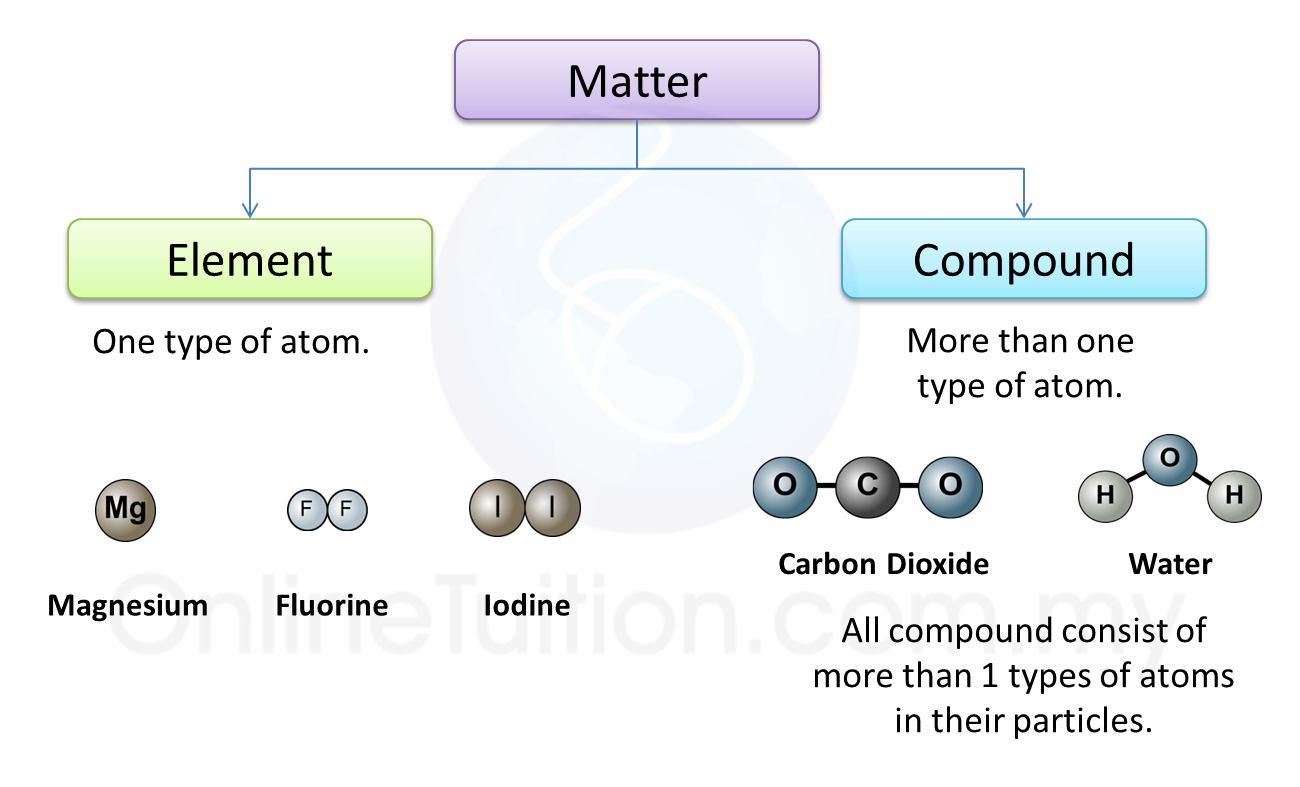
1. Molecular substances are made up of molecules which have two or more atoms of the same type or different types.
2. For example, two oxygen atoms combine to form one oxygen molecule. On the other hand, one nitrogen atom combines with three hydrogen atoms to form one ammonia molecule.
3. Examples of other molecular substances are hydrogen, nitrogen, carbon dioxide, sulphur dioxide, chlorine gas and iodine.
4. Molecules are held together by weak intermolecular force (or van der Waals forces) and have low melting and boiling points.
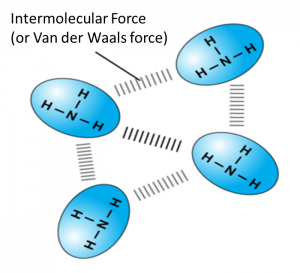
5. Molecules are made up of non-metal atoms which are poor conductors of electricity because there are no free electrons or ions in any state to carry electric charge.
Substances made of ions (or Ionic substances)
1. Substances that made up of ions are called the ionic compounds.
2. They are compounds of metal bonded with non-metal.
3. Examples of ionic compounds are sodium chloride, copper (II) sulphate, sodium hydroxide and lead (II) oxide.
4. Positive and negative ions are held together by strong ionic bonds. This strong bonding force makes ionic compounds has high melting and boiling points.
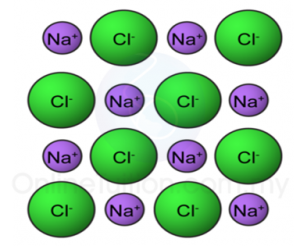
Arrangement of ions in a compound
5. All ionic compounds are crystalline solids at room temperature.
6. The solid crystals do not conduct electricity because the ions are not free to move to carry an electric current.
7. However, if the ionic compound is melted or dissolved in water, the liquid will now conduct electricity, as the ion particles are now free.
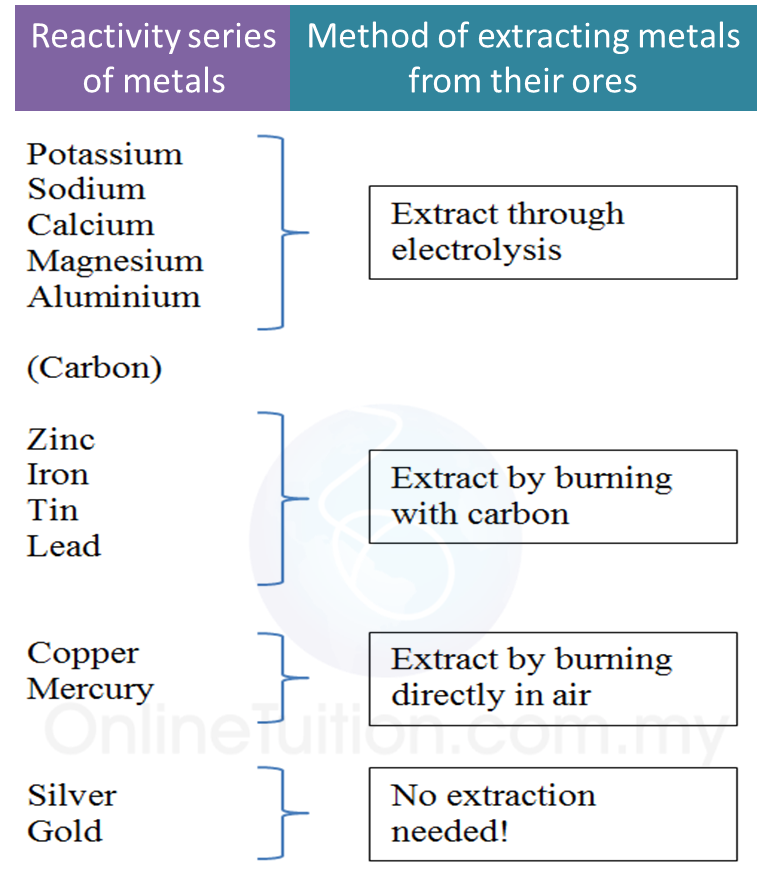
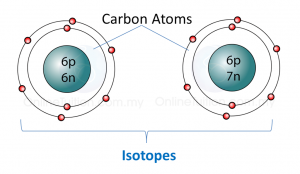
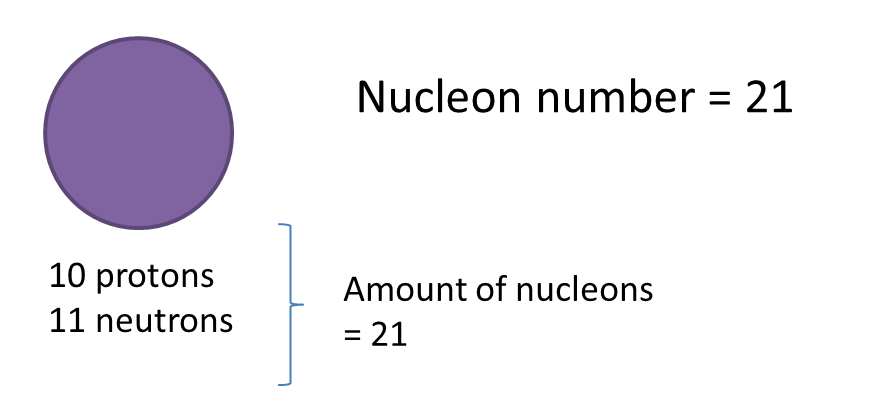
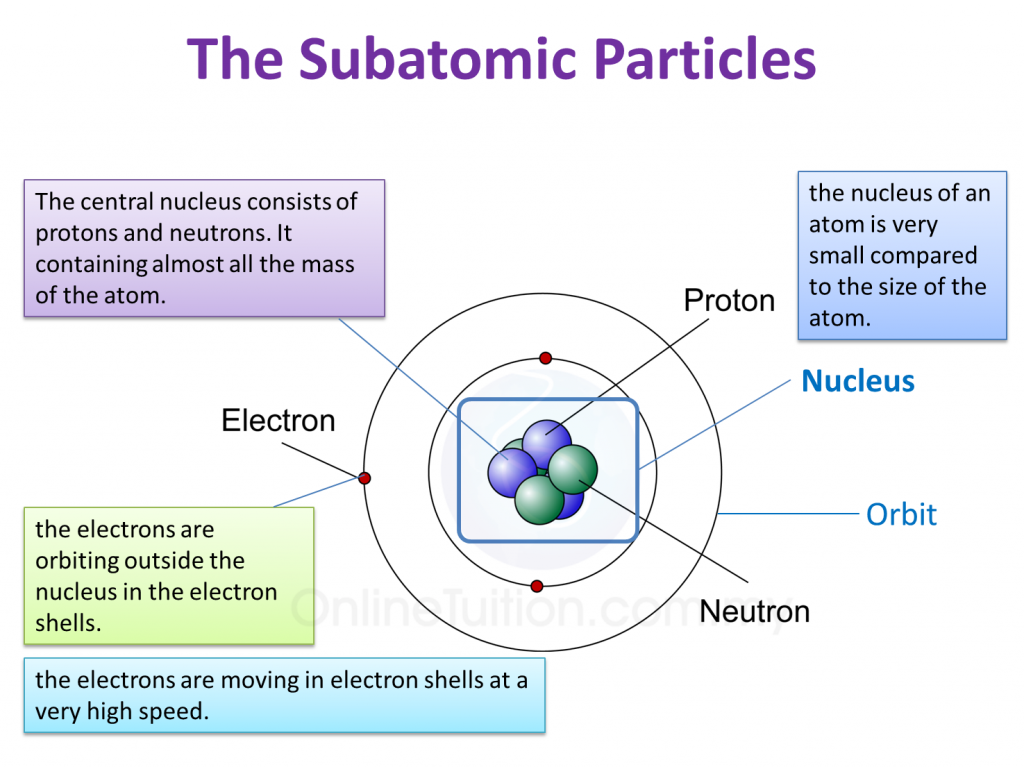
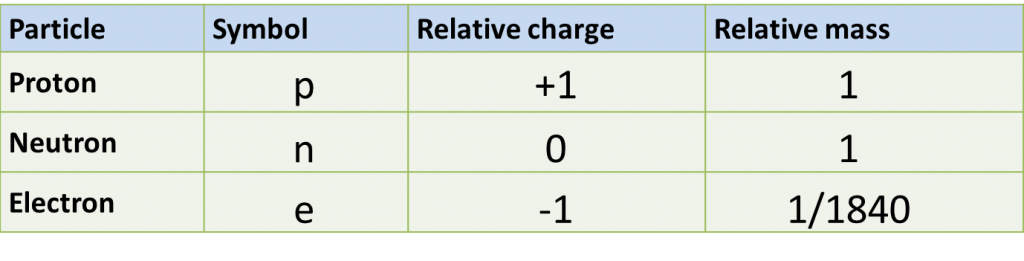
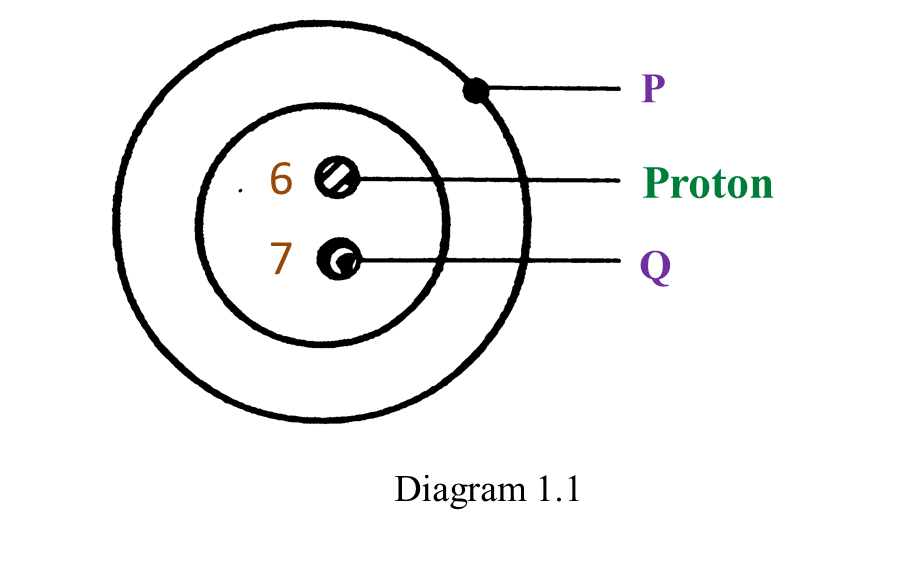 (a) P and Q are subatomic particles.
(a) P and Q are subatomic particles.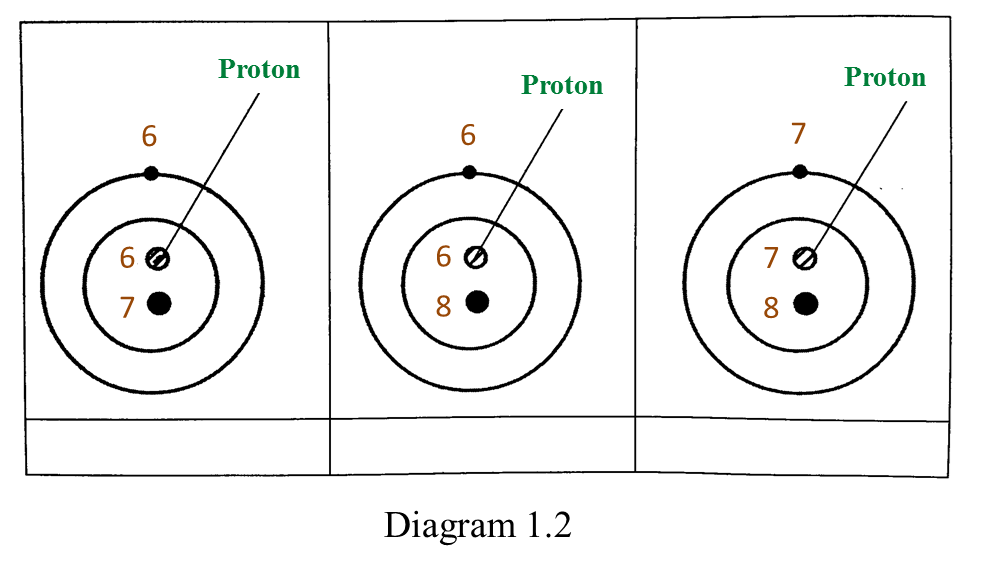
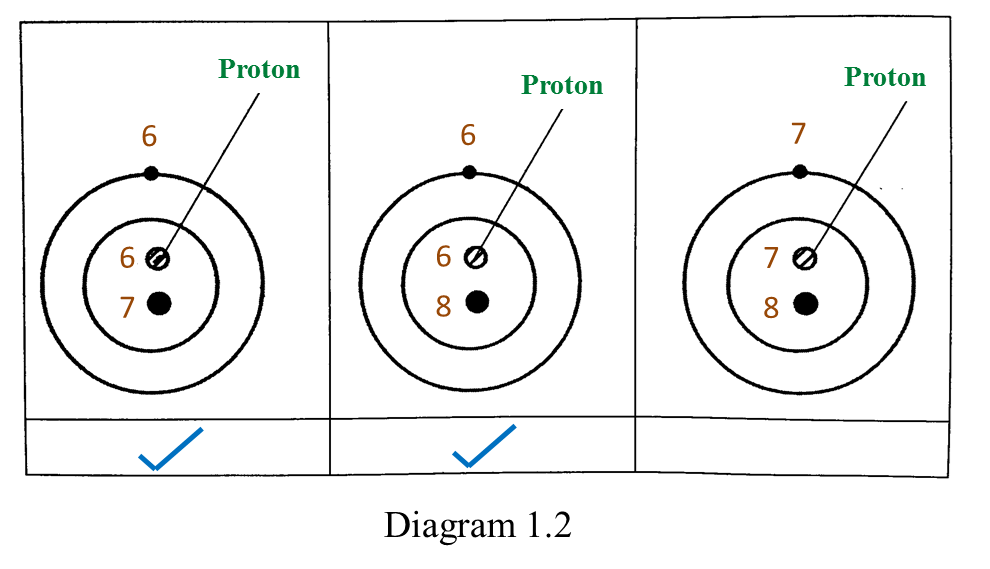
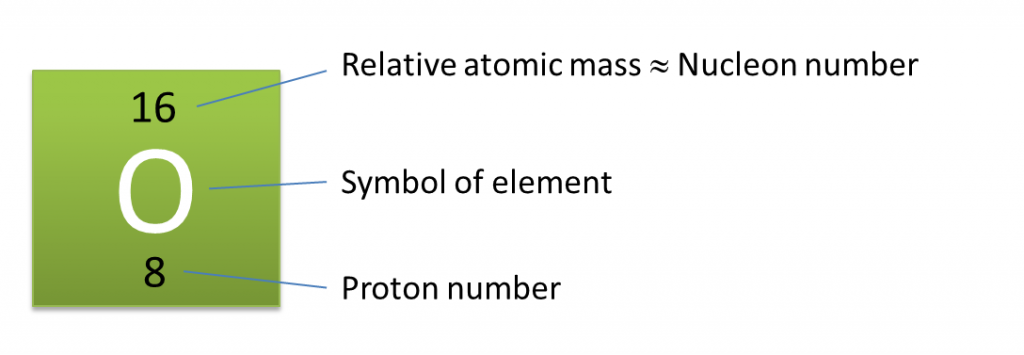
 Based on the Diagram,
Based on the Diagram,