Change in Heat and Kinetic Energy of Particles
1. The change in temperature will influences the kinetic energy or the speed of the motion of the particles.2. When a substance is heated, the kinetic energy of the particles in the substance increases. This causes the particles to move or vibrate faster.
3. Likewise, when a substance is cooled, the kinetic energy of the particles in the substance decreases. This causes the particles to move or vibrate slower.
4. The kinetic energy of the particles in a substance is directly proportional to the temperature of the substance.
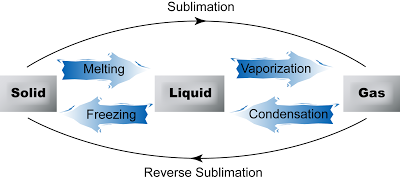
Inter-conversion between States of Matter
| Melting | Definition Melting is the process where a solid changes to its liquid state at a certain temperature (called the melting point) and pressure when it is heated. Notes
|
| Freezing | Definition Freezing is the process where a liquid changes to its solid state at a certain temperature (called freezing point) and pressure when it is cooled. Notes
|
| Vaporization (Evaporation) |
Definition Vaporization, also called evaporation is the process whereby atoms or molecules in a liquid state gain sufficient energy to enter the gaseous state. Boiling is the rapid vaporization of a liquid at a certain temperature (the boiling point) and pressure when heat is applied to it. Notes Evaporation
|
| condensation | Definition Condensation is the process by which a gas or vapor changes to liquid state at certain temperature and pressure when it is cooled. Notes
|
| sublimation | Definition Sublimation is a process of conversion of a substance from the solid to the vapour state without its becoming liquid. Notes
|
Interesting Video:
Inter-conversion Between the 3 States of Matter











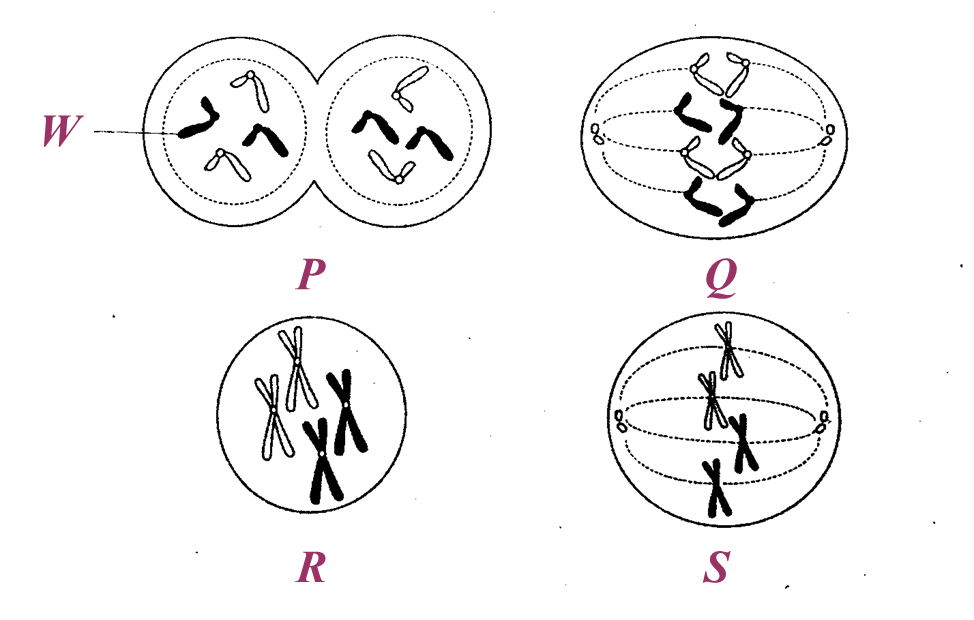

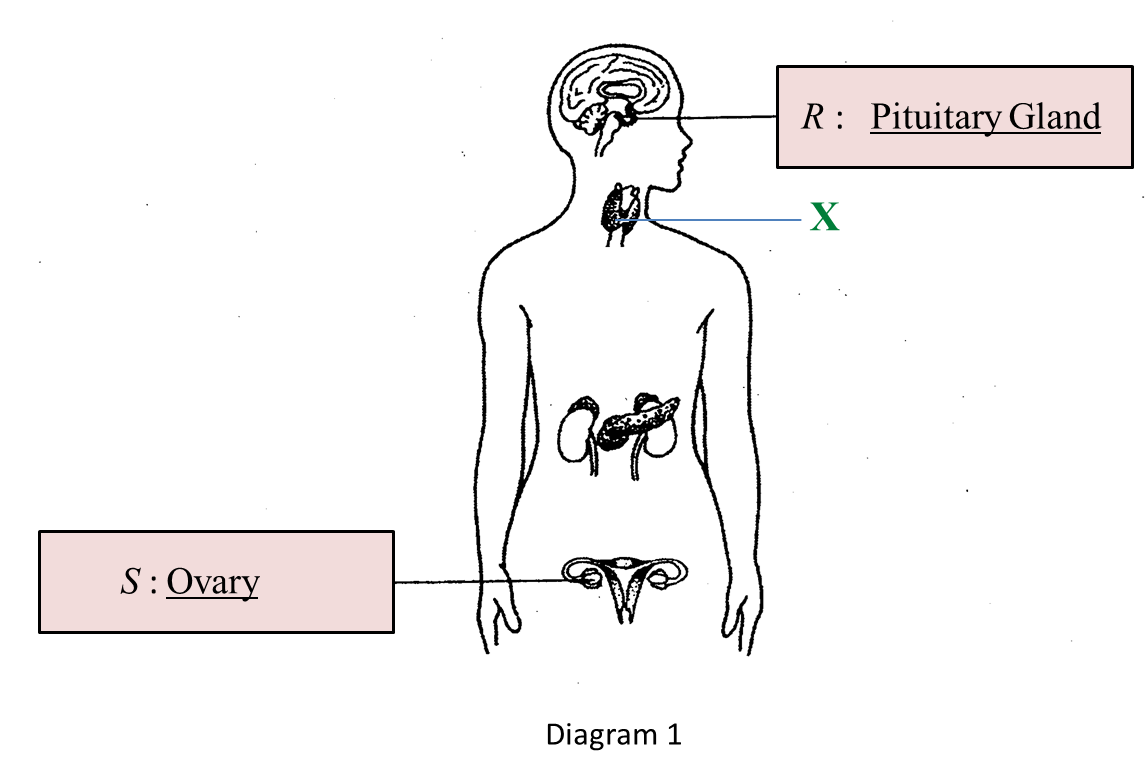
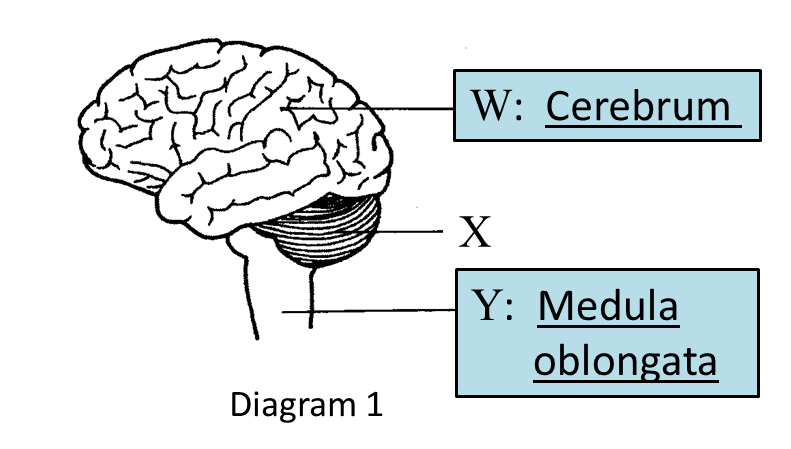
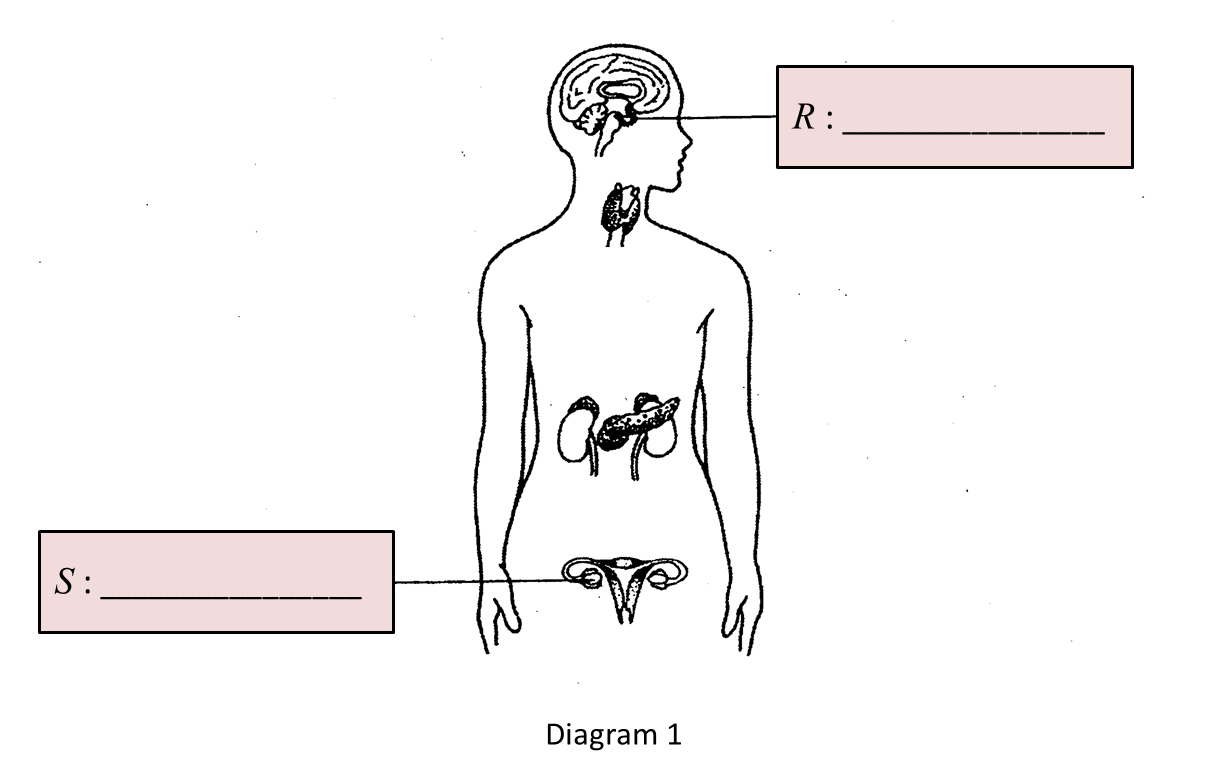 (a) Name glands R and S in the boxes provided in Diagram 1.
(a) Name glands R and S in the boxes provided in Diagram 1.