[adinserter block="3"]
Question 1:
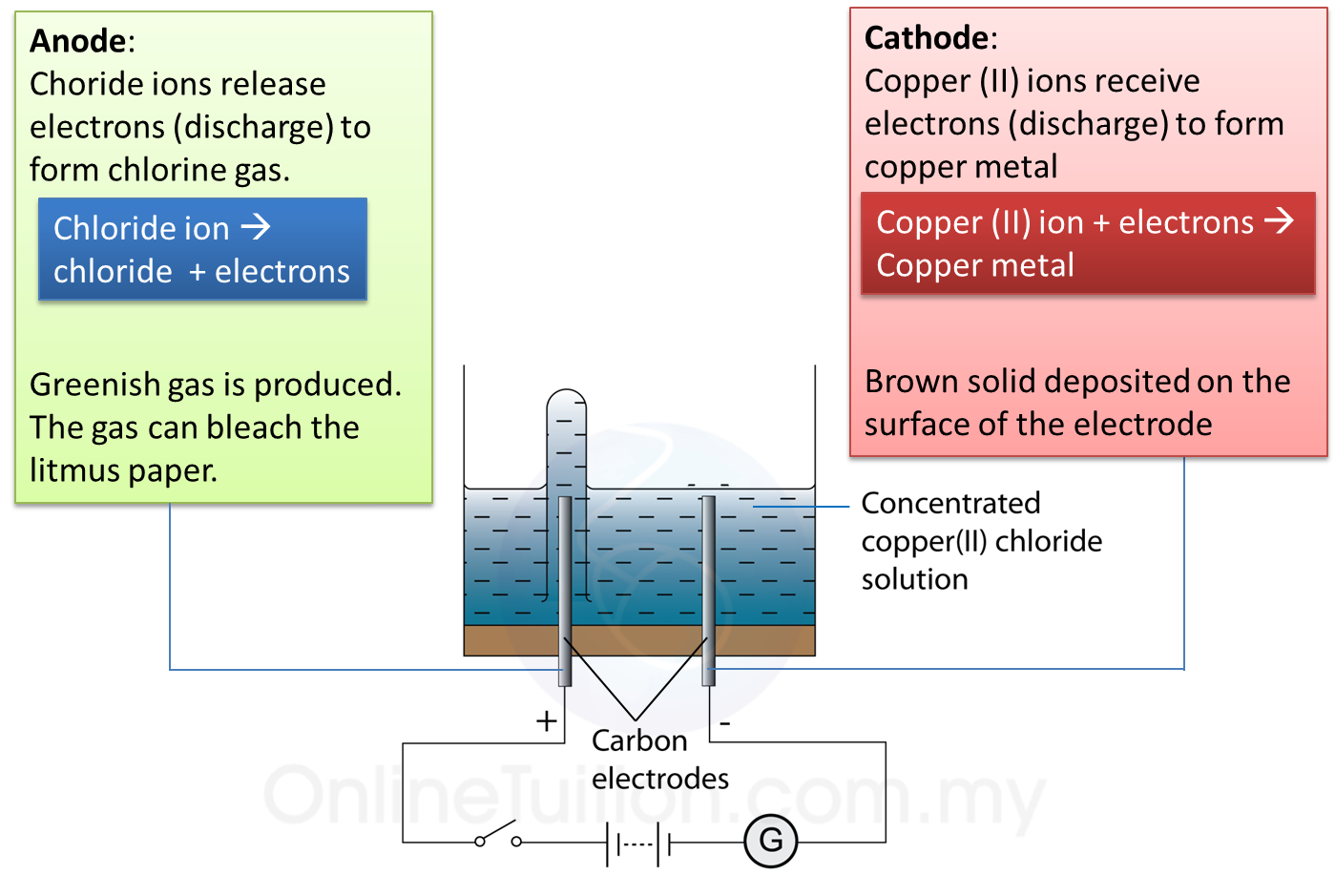
Figure I represents a model of the human lungs in the respiratory mechanism.
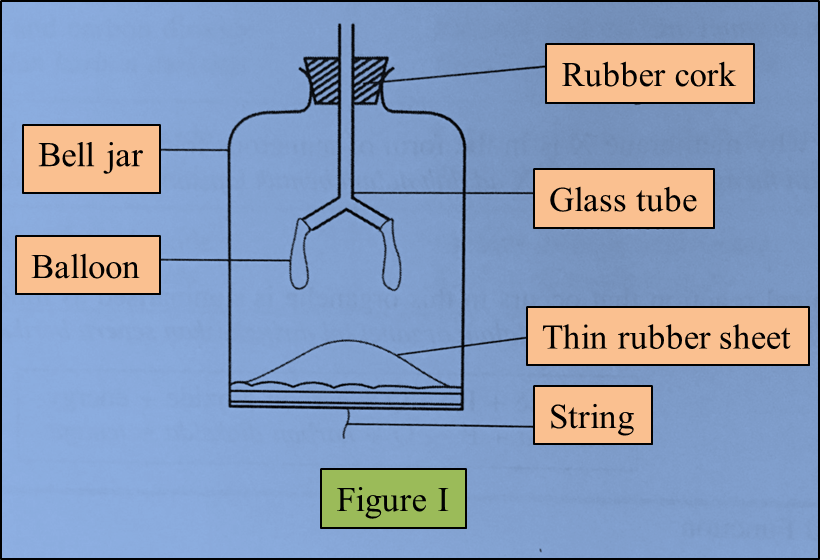
(a) Based on the model of the lungs in Figure I, what are the equivalent structures to the glass tube and the bell jar in the human respiratory system?
Glass tube: __________
Bell jar: ____________
(2 marks)
(b)(i) The thin rubber sheet represents the diaphragm in the human respiratory system.
What is the function of the thin rubber sheet in the model of the lungs? (1 mark)
(ii) The balloons represent the human lungs.
Explain one characteristic of the balloons which is similar to the human lungs. (2 marks)
[adinserter block="3"]
(c)(i) The string in the model of the lungs is pulled down.
Draw the changes to the thin rubber sheet and the balloons in Figure II below. (1 mark)
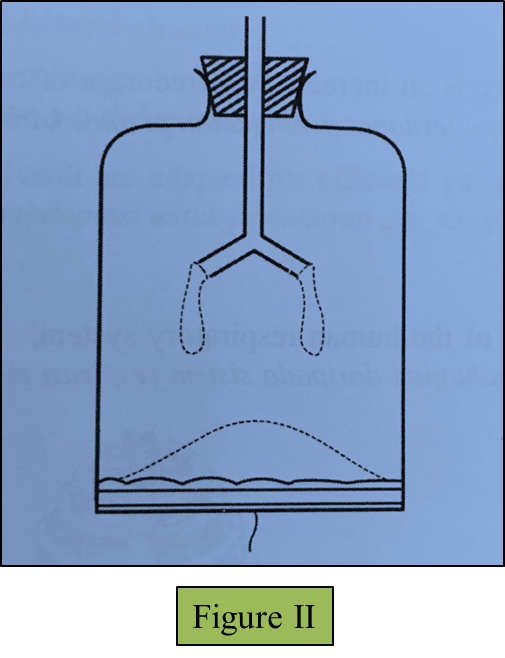
(ii) Observe your drawing in (c)(i).
Explain the relationship between the changes in the model of the lungs you have drawn and the real human respiratory system. (3 marks)
[adinserter block="3"]
(d)(i) The percentage of oxygen and carbon dioxide gases in inspired and expired air is determined by using the J-tube.
Why is the end of the J-tube dipped in potassium hydroxide solution and then followed by potassium pyrogallol solution? (1 mark)
(ii) Table below shows the result of a study on the content of inspired and expired air.
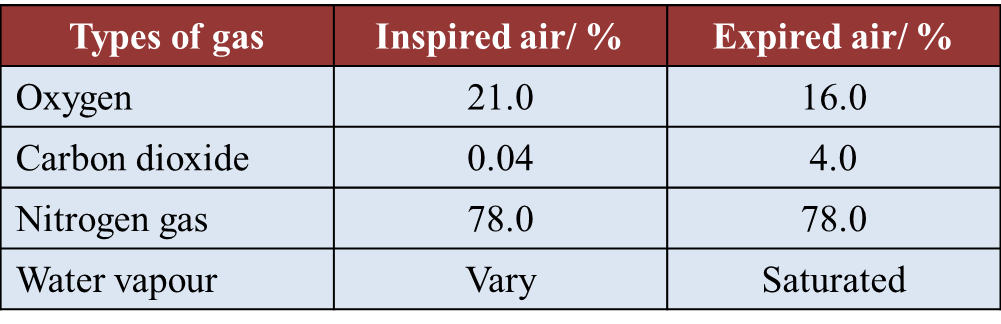
Explain why there is an increase in percentage of carbon dioxide in the expired air. (2 marks)
[adinserter block="3"]
Answer:
(a)
Glass tube: Bronchi/ trachea
Bell jar: Rib cage/ ribs
(b)(i)
To control the volume/ pressure of the air in the bell jar
(b)(ii)
Balloons are elastic and can expand and contract
(c)(i)
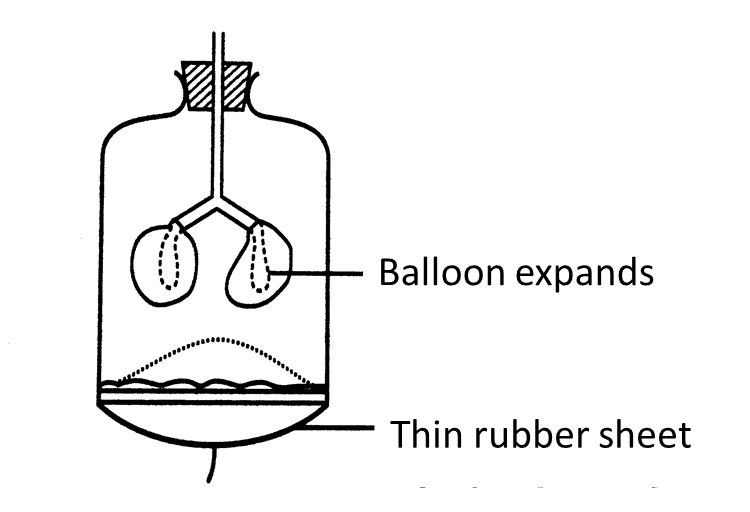
[adinserter block="3"]
(c)(ii)
The diaphragm muscles contract causing the volume of the thoracic cavity to increase. This causes the pressure in the lungs to decrease. Air from the outside is forced in.
(d)(i)
Potassium hydroxide solution absorbs carbon dioxide only whereas potassium pyrogallol solution absorbs both oxygen and carbon dioxide.
(d)(ii)
Carbon dioxide is the product of cellular respiration in the body. Carbon dioxide diffuses out of the cells to be transported to the lungs.
[adinserter block="3"]
Figure I represents a model of the human lungs in the respiratory mechanism.

(a) Based on the model of the lungs in Figure I, what are the equivalent structures to the glass tube and the bell jar in the human respiratory system?
Glass tube: __________
Bell jar: ____________
(2 marks)
(b)(i) The thin rubber sheet represents the diaphragm in the human respiratory system.
What is the function of the thin rubber sheet in the model of the lungs? (1 mark)
(ii) The balloons represent the human lungs.
Explain one characteristic of the balloons which is similar to the human lungs. (2 marks)
[adinserter block="3"]
(c)(i) The string in the model of the lungs is pulled down.
Draw the changes to the thin rubber sheet and the balloons in Figure II below. (1 mark)

(ii) Observe your drawing in (c)(i).
Explain the relationship between the changes in the model of the lungs you have drawn and the real human respiratory system. (3 marks)
[adinserter block="3"]
(d)(i) The percentage of oxygen and carbon dioxide gases in inspired and expired air is determined by using the J-tube.
Why is the end of the J-tube dipped in potassium hydroxide solution and then followed by potassium pyrogallol solution? (1 mark)
(ii) Table below shows the result of a study on the content of inspired and expired air.

Explain why there is an increase in percentage of carbon dioxide in the expired air. (2 marks)
[adinserter block="3"]
Answer:
(a)
Glass tube: Bronchi/ trachea
Bell jar: Rib cage/ ribs
(b)(i)
To control the volume/ pressure of the air in the bell jar
(b)(ii)
Balloons are elastic and can expand and contract
(c)(i)

[adinserter block="3"]
(c)(ii)
The diaphragm muscles contract causing the volume of the thoracic cavity to increase. This causes the pressure in the lungs to decrease. Air from the outside is forced in.
(d)(i)
Potassium hydroxide solution absorbs carbon dioxide only whereas potassium pyrogallol solution absorbs both oxygen and carbon dioxide.
(d)(ii)
Carbon dioxide is the product of cellular respiration in the body. Carbon dioxide diffuses out of the cells to be transported to the lungs.
[adinserter block="3"]
Question 2:
(a) Sugar is oxidized during respiration.
Explain the process. (4 marks)
(b) After an athlete finished running a race, his breathing is still fast and deep for several minutes. (6 marks)
Explain why.
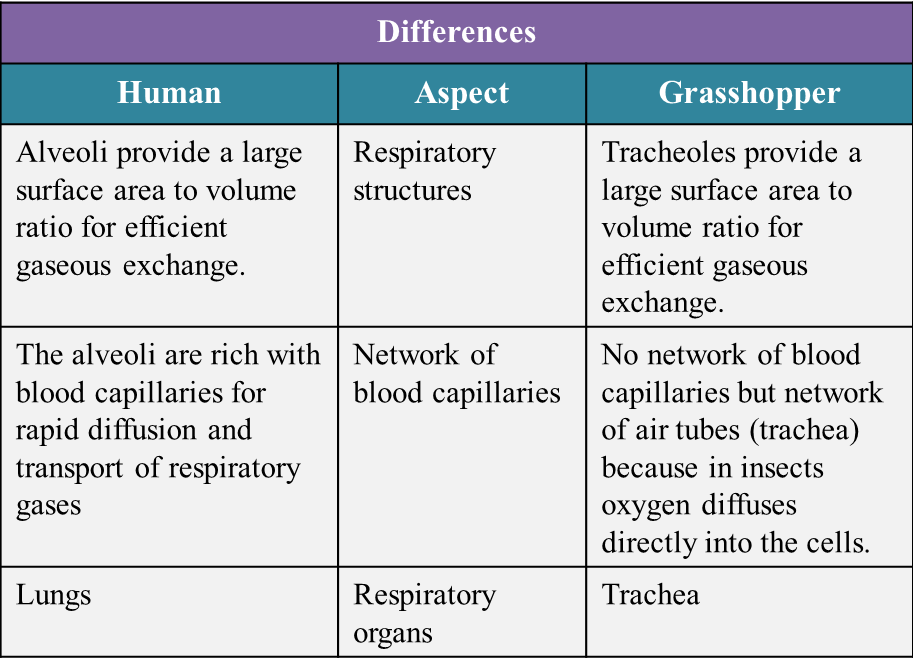
(c) State the similarities and differences between the human and the grasshopper’s respiratory system based on the structural adaptation to maximize the rate of gaseous exchange. (10 marks)
[adinserter block="3"]
Answer:
(a)
- During respiration, oxygen taken in is transported by the blood circulatory system to the body cells.
- In the cells, sugar (glucose molecules) are oxidized by the oxygen to release energy in the form of ATP.
- Other products of this oxidation process are carbon dioxide and water.
[adinserter block="3"]
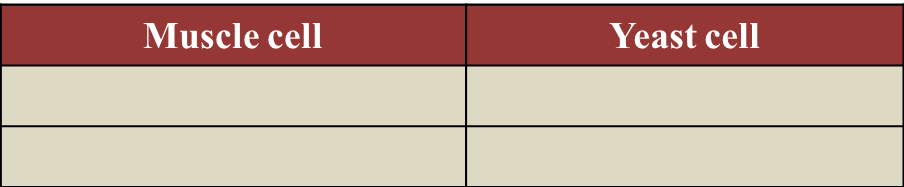
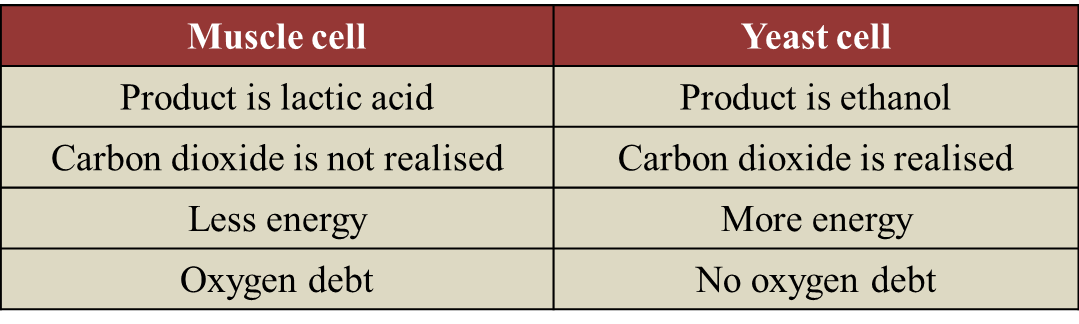
(b)
- He needs to breathe in fast and deep in order to inhale more oxygen.
- This is because during running, the rate of oxygen being used by the muscles exceeds the amount of oxygen supplied by the blood.
- The muscles are in a state of oxygen deficiency.
- An oxygen debt is incurred.
- The muscles obtain the extra energy (ATP) from anaerobic respiration, because oxygen is not available.
- The excess oxygen inhaled is used by the body to oxidise the accumulated lactic acid to carbon dioxide and water.
- When all the lactic acid is removed, the oxygen debt is paid off.
[adinserter block="3"]
(c)
Similarities:
- Both respiratory systems have structures which have a large surface area to volume ratio for efficient gaseous exchange.
- The cells that lining the respiratory structures are thin. This allows gas diffusion to take place efficiently.
- The surfaces for gaseous exchange are constantly moist because they are covered by a film of water which allows the respiratory gases to dissolve in it.
(a) Sugar is oxidized during respiration.
Explain the process. (4 marks)
(b) After an athlete finished running a race, his breathing is still fast and deep for several minutes. (6 marks)
Explain why.
(c) State the similarities and differences between the human and the grasshopper’s respiratory system based on the structural adaptation to maximize the rate of gaseous exchange. (10 marks)
[adinserter block="3"]
Answer:
(a)
- During respiration, oxygen taken in is transported by the blood circulatory system to the body cells.
- In the cells, sugar (glucose molecules) are oxidized by the oxygen to release energy in the form of ATP.
- Other products of this oxidation process are carbon dioxide and water.
[adinserter block="3"]
(b)
- He needs to breathe in fast and deep in order to inhale more oxygen.
- This is because during running, the rate of oxygen being used by the muscles exceeds the amount of oxygen supplied by the blood.
- The muscles are in a state of oxygen deficiency.
- An oxygen debt is incurred.
- The muscles obtain the extra energy (ATP) from anaerobic respiration, because oxygen is not available.
- The excess oxygen inhaled is used by the body to oxidise the accumulated lactic acid to carbon dioxide and water.
- When all the lactic acid is removed, the oxygen debt is paid off.
[adinserter block="3"]
(c)
Similarities:
- Both respiratory systems have structures which have a large surface area to volume ratio for efficient gaseous exchange.
- The cells that lining the respiratory structures are thin. This allows gas diffusion to take place efficiently.
- The surfaces for gaseous exchange are constantly moist because they are covered by a film of water which allows the respiratory gases to dissolve in it.


 (i) Name gas R.
(i) Name gas R.